In it’s 5th and final year of funding, the CRC connects, in a highly collaborative setting, 19 PI with unique expertise across all research areas. In 13 CRC research projects, they reach beyond the constraints of any individual target listed above, linking sub- and supra-cellular expertise, tools and techniques. The non-canonical nature of research targets, project design, and team interactions are seen as a major asset of the consortium, as it improves the ability to identify and validate relevant biological behaviour at molecular, cellular, and organ levels – to inform clinical translation.
Research Areas |
||
A: Heterogeneous Cardiac Cell Types/ Activation States |
B: The Heterocellular Nature of Cardiac Lesions |
C: Steering Heterocellular Cross-Talk in Cardiac Lesions |
Identities
|
Interactions
|
Implications
|
Markers and Modulators
|
Steering Tools
|
Translation
|
Service Projects |
||
S1: Advanced Fluorescence Imaging |
S2: CardioVascular BioBank |
S3: Sequencing and Bioinformatics |
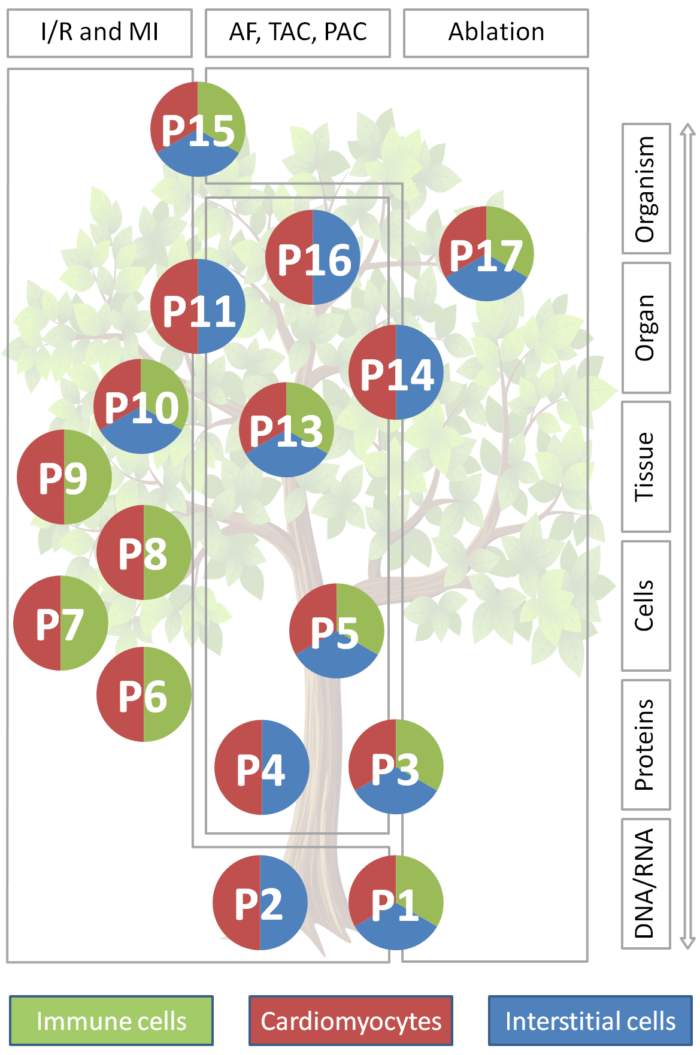
Traditionally, heart research has focussed on cardiac myocytes: they are pivotal for cardiac function and their activity drives classic clinical read-outs such as blood pressure or ECG. Also, cardiomyocytes occupy about two thirds of myocardial volume. But: non-myocytes – including interstitial and immune cells such as fibroblasts and macrophages, as well as endothelial cells, adipocytes, and neurons – contribute about two thirds to cardiac cell numbers, even in healthy myocardium. This majority-fraction is further enriched in tissue lesions. Non-myocytes are increasingly seen as key determinants of cardiac structural and functional integration in homeostasis and disease, including direct electrical cross-talk of fibroblasts and of macrophages with cardiomyocytes. This highlights non-myocytes as potential targets for therapeutic intervention, in addition to (not instead of) cardiomyocytes. Indeed, scarring and fibrosis – read-outs primarily of altered non-myocytes activity – contribute to heart failure and are independent predictors of heart rhythm disturbances and sudden death. In line with this, several therapies that reduce arrhythmogenesis and/ or progression of heart diseases are known to affect, in part at least, non-myocytes (e.g. ACE inhibitors, AT1- and β-blockers, MR antagonists, statins).
Our knowledge about non-myocytes cellular identities, mechanisms of heterocellular cardiomyocyte-non-myocyte and non-myocyte-non-myocyte interactions, and the implications of such heterocellular cross-talk for the heart is still in its infancy. The CRC intends to advance this field by comprehensively characterising these identities, interactions, and implications, with focus on tissue remodelling in cardiac lesions. This requires:
- a multi-level research approach, from molecular characterisation of cell identities and states, to cell-interaction assays, and intervention models at cell / tissue / organ / organism levels;
- a multi-parametric technical approach using an extensive range of methods to characterise, and subsequently alter, heterotypic cell and interaction properties at all of the above levels;
- a multi-dimensional data mapping design for interrelating information gleaned in 3D space from nano- to macro-scales, in time from ms to months, and in species from mouse to man;
- a multi-disciplinary consortium of experts with backgrounds from epigenetics, molecular and cell biology, to biophysics, engineering, (patho-)physiology, pharmacology and clinical practice;
- a unifying conceptual framework and suitable consolidating structure to serve as an umbrella under which the above can come together to effectively address one common goal: taking knowledge of cardiac structure and function beyond the myocyte-centric view of the heart.
Appreciation of the heterocellular nature of complex tissues as a pre-condition for understanding organ function is of principal relevance beyond the heart (e.g. ‘new roles’ for glia in the nervous system). The CRC will improve the understanding of cardiac non-myocyte-cardiomyocyte interactions in general, and in tissue lesions in particular. In the longer term, the CRC will establish new approaches for addressing prominent clinical challenges by influencing structural and functional properties of focal lesions such as post-infarct or post-ablation scars, and global remodelling in atrial or ventricular overload. The long-term vision builds on solid basic science to develop the tools and knowledge needed for steering heterocellular communication for medical benefit. This is hoped to give rise to a principally new take on prevention, management, and therapy of diseases of the heterocellular heart.
The CRC concept is distinct from, yet compatible with, efforts to regenerate cardiac muscle: to work with nature’s own repair processes ‘to make better scars’ – i.e. scars that serve their mechano-structural repair function while causing fewer side effects, from fibrosis to arrhythmias.
This requires:
- a thorough understanding of the various cell populations and their activation states relevant for myocardial homeostasis and injury-related remodelling: Identities
- insight into heterocellular non-myocyte-non-myocyte and cardiomyocyte-non-myocyte cross-talk involved in cardiac homeostasis and responses to injury: Interactions
- development of means for targeting NM to steer lesion properties, maintaining their pathophysiological benefit while reducing ‘side effects’, such as arrhythmogenicity: Implications
Research is supported by three scientific service projects, each required for a majority of constituent CRC proposals: one focussed on advanced fluorescence imaging (S1), one on providing access to human tissue, associated clinical/ wet-lab data and support for large animal models (S2), and one on supporting sequencing and bioinformatics (S3). Data handling across the CRC is based on the infrastructure established by the INF project during year 1-4, and is now coordinated by a Data Steward in year 5.








